Information about the Corona pandemic

Sunday, February 1st, 2026Vaccination – the most effective invention in medical history
Up to 3 million children worldwide died of measles each year before the measles vaccine was introduced in the 1960s2. Today, complete measles vaccination prevents 97% of cases of measles3.
We are unaware of these amazing successes because, thanks to vaccinations, we hardly ever see infections and their cruel consequences anymore. We no longer experience half of our siblings dying in childhood from measles, whooping cough, polio, tetanus, and other diseases. That is precisely why we need to make a conscious effort to remember what vaccinations achieve.
Treating infections is good, but preventing illness in the first place is even better. Vaccinations can do just that.
In the following, I will address the following questions:
1. How many people died from common infectious diseases before vaccinations were available?
2. How many cases of illness and death have vaccinations prevented?
3. What is the relationship between the benefits of vaccinations and their possible side effects?
1. How many people died from common infectious diseases before vaccinations were available?
Smallpox was one of the deadliest viruses in human history. Globally, 300-500 million people died of smallpox in the 20th century1 (for comparison: Europe today has a population of around 500 million).
Before the introduction of vaccination, measles caused 2-3 million deaths worldwide each year. After vaccination was introduced in the 1960s, both the incidence and mortality rates declined dramatically. Complete vaccination (2 vaccinations) prevents disease in 97% of cases.
2. How many cases of illness and death have vaccinations prevented?
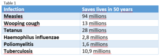
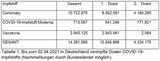
Between 1974 and 2024, vaccinations prevented approximately 154 million deaths from 14 diseases4.
Table 1 shows how many lives vaccinations have saved over the past 50 years, including 94 million lives saved from measles, 13 million from whooping cough, and 28 million from tetanus4.
In addition, there are numerous cases of permanent health damage among survivors, e.g., permanent paralysis after polio (infantile paralysis) or brain damage after measles.
A particularly striking example is smallpox: in 1900, two 13-year-old students in the same class contracted smallpox from the same student. The unvaccinated student suffered a disfiguring smallpox infection, while the other had hardly any symptoms. The picture speaks volumes, but is unpleasant to look at, so please only view it if you can stomach it: View here, Fig. 8.
For the years 2021-2030, there are projections that consistent vaccination could save a total of 50 million lives.
3. What is the relationship between the benefits of vaccinations and their possible side effects?
- A vaccine reaction is a sign that the immune system has been activated. Typically, arm pain at the injection site, possibly accompanied by slight swelling, as well as fatigue, aching limbs, headaches, and possibly fever, are observed. However, you do not necessarily have to have these symptoms to know that the vaccination is working.
- On the other hand, a cold after a flu vaccination would not primarily be considered a vaccine reaction or side effect, but rather a normal cold that would have occurred even without the vaccination.
Practical example:
If you attend a school with approximately 1,000 students, vaccination prevents the deaths of 6-7 students at that school. Without vaccination, there would have been one death in approximately every second grade.
With vaccination, no students would have died, but statistically, no students would have suffered side effects either.
If you consider all schools in Wiesbaden (school year 2024/2025: 33,433 students13), only 1-2 students in the whole of Wiesbaden would have suffered from thrombocytopenia, i.e., a lack of blood platelets (which is easily treatable).
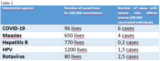
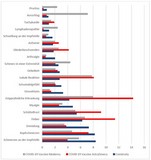
Table 2 shows an overview of vaccinations and the lives saved per 100,000 vaccinations, as well as the average number of side effects observed among 100,000 vaccinated individuals6-12.
In my entire career as a family doctor, I have administered around 18,000 vaccinations, and in all that time there has not been a single serious side effect from a vaccination.
On the other hand, I have unfortunately seen a lot of suffering, including deaths, as a result of infectious diseases that could have been prevented by vaccination.
In summary, it is a matter close to my heart to protect you from suffering and worse through vaccinations. If you have any questions about this, I am always available to talk to you.
References
1 Berche P. Life and death of smallpox. Presse Med. 2022 Sep;51(3):104117
2 Peltola H. The History of Measles and Vaccine Development. Acta Paediatr. 2025 Nov 17
3 Ghosh S, Kappara D, Majumder N, Nath-Sain S, Roy AD, Mukhopadhyay S. A systematic review and modelling insights of factors impacting measles vaccine effectiveness, efficacy and immunogenicity. Discov Viruses. 2025;2(1):23. doi: 10.1007/s44370-025-00027-8. Epub 2025 Aug 28. PMID: 40918614; PMCID: PMC12412780.
4 Shattock AJ et al. Contribution of vaccination to improved survival and health: modelling 50 years of the Expanded Programme on Immunization. Lancet. 2024 May 25;403(10441):2307-2316
5 Carter A et al. Modeling the impact of vaccination for the immunization Agenda 2030: Deaths averted due to vaccination against 14 pathogens in 194 countries from 2021 to 2030. Vaccine. 2024 Apr 8;42 Suppl 1:S28-S37
6 Steele MK et al. Estimated Number of COVID-19 Infections, Hospitalizations, and Deaths Prevented Among Vaccinated Persons in the US, December 2020 to September 2021. JAMA Netw Open. 2022;5(7):e2220385. doi:10.1001/jamanetworkopen.2022.20385
7 Cappelletti-Montano B et al. A comparative analysis on serious adverse events reported for COVID-19 vaccines in adolescents and young adults. Front Public Health. 2023 Jun 12;11:1145645
8 Jaspreet Toor et al (2021). Lives saved with vaccination for 10 pathogens across 112 countries in a pre-COVID-19 world eLife 10:e67635
9 Ghosh S, Kappara D, Majumder N, Nath-Sain S, Roy AD, Mukhopadhyay S. A systematic review and modelling insights of factors impacting measles vaccine effectiveness, efficacy and immunogenicity. Discov Viruses. 2025;2(1):23. doi: 10.1007/s44370-025-00027-8. Epub 2025 Aug 28. PMID: 40918614; PMCID: PMC12412780.
10 Gong X, Fang Q, Zhong J, Zheng C, Yin Z. Adverse event reporting following immunization of hepatitis B vaccine: A 13-year review. Hum Vaccin Immunother. 2024 Dec 31;20(1):2411824. doi: 10.1080/21645515.2024.2411824. Epub 2024 Oct 13. PMID: 39396824; PMCID: PMC11485979.
11 Cappelletti-Montano B et al. A comparative analysis on serious adverse events reported for COVID-19 vaccines in adolescents and young adults. Front Public Health. 2023 Jun 12;11:1145645.
12 Maglione MA et al. Safety of Vaccines Used for Routine Immunization in the United States. Evid Rep Technol Assess (Full Rep). 2014 Jul;(215):1-740
13 https://www.wiesbaden.de/leben-in-wiesbaden/stadtportraet/daten-fakten/jb-bildung_und_ausbildung
Sunday, August 17th, 2024
COVID vaccination: Yes or no?
The key advantages and disadvantages of COVID vaccination are outlined below to help you make the right choice for your health.
Some of the statements have been simplified to make them easier to understand, but the essence remains the same. More detailed information can be found in the cited articles under the references.
The data is up to date and the information in this article relates to fully vaccinated people and to the Omicron variant of the SARS-CoV-2 virus.
In unvaccinated people, however, the risks of COVID infection are significantly greater and the benefits of vaccination are correspondingly much higher.
For earlier virus variants, especially the delta virus, the risks of COVID infection are also significantly higher and the benefits of vaccination are correspondingly much greater.
1. Risks also remain for fully vaccinated people.
Fully vaccinated people have a good cellular defense, which means that they can produce antibodies against a COVID infection within a few days, but by then the disease may have already caused a severe course.
The booster vaccination provides fresh antibodies for about 6 months, so that antibodies are available immediately from the moment of a COVID infection and the course is significantly mitigated.
This is the reason why a new COVID vaccination is necessary every year before the corona wave, which usually occurs in the fall, even if it is already the hundredth.
The risks of COVID infection are as follows:
1. Long-COVID1, i.e. loss of physical performance, usually in the form of severe physical exhaustion, often resulting in partial or complete incapacity to work for months. In some cases, early retirement is even the result.
2. Hospitalization, i.e. a course so severe that the patient has to be admitted to hospital.
3. Death due to COVID.
The respective risks can be roughly estimated as a percentage, but are not yet precise as studies are still ongoing. However, a reasonable estimate can already be given, whereby I tend to indicate the more “optimistic” end of the risk range2:
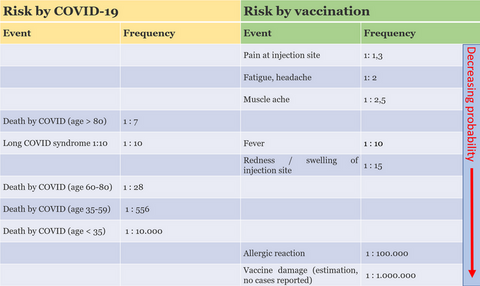
1. The risk of a long-COVID infection is around 5%, i.e. 1:20.
2. The risk of hospitalization due to COVID infection is approx. 1%, i.e. 1:100.
3. The risk of a fatal outcome of a COVID infection is approx. 0.1%, i.e. 1:1,000.
COVID vaccination has risks, but these are very low and usually curable3.
The risks of COVID vaccination are inflammation of the heart muscle (myocarditis), allergic reactions (anaphylaxis) and thrombosis. However, no link has been established with Guillain-Barré syndrome, facial nerve paralysis (facial nerve palsy) or deaths.
1. Heart muscle inflammation (myocarditis): The risk is less than 1 in 10,000 for men up to the age of 29. The risk is significantly lower for all others. In over 60s, the risk is less than 1:100,000. With appropriate treatment, myocarditis heals in almost all cases and leaves no damage.
2. Allergic reactions (anaphylaxis): the risk is around 1:100,000 and occurs within 15-30 minutes. The allergic reaction can be treated immediately and effectively with appropriate medication.
3. Thrombosis (TTS, thrombosis-thrombocytopenia syndrome): This risk is around 1:100,000 with the so-called “Astra Zeneca” vaccine (Vaxzevria), which is no longer available in Germany. With the mRNA vaccines (“Biontech and Moderna”), no such risk could be proven or is so low that it cannot be proven statistically.
Disinformation on the internet about other side effects is either non-existent or so extremely rare that it cannot be proven and is negligible compared to the risks of COVID infection and therefore negligible.
Psychology plays a huge role: we have to actively choose to be vaccinated and therefore reasonably look at the risks of vaccination.
When it comes to infection, however, we forget to look at the risks and are happy to ignore the fact that we will be infected at some point. However, we will inevitably be infected with COVID not just once but repeatedly in the coming years, some of us even several times a year.
This is why a sober comparison of the risks of both events, i.e. COVID infection and vaccination, is so crucial.
Summary:
The risks of COVID infection are significantly greater than those of vaccination.
The risk of a person over 60 years of age dying from COVID is around 100 times higher than the risk of a (treatable) allergic reaction or other side effects.
The risk of needing hospital treatment for COVID infection is even 1000 times higher than the risk of any potential side effects of COVID vaccination.
And the risk of Long COVID is probably 5000 times higher than the risk of vaccine side effects.
References:
1. Homepage of the Long COVID Initiative of the Federal Ministry of Health. Link
2. Arabi M. et al: Severity of the Omicron SARS-CoV-2 variant compared with the previous lineages: A systematic review. J Cell Mol Med. 2023 Jun;27(11):1443-1464. Link
3. Paul-Ehrlich-Institut: Safety profile of COVID-19 vaccines - status as of March 31, 2023. Link
4. Epidemiological Bulletin No. 21/2023 of May 25, 2023. Link
Sunday, August 17th, 2024COVID vaccination in 2024: yes or no?
Let's ask Chat GPT! (“Artificial intelligence”)
Let's ask Chat GPT! (“Artificial intelligence”)
“What percentage of COVID infections with Omicron lead to hospitalizations?”
Answer Chat GPT:
“The hospitalization rate for COVID-19 infections varies by variant, vaccination status, age, pre-existing conditions and other factors. There are some specific observations for the Omicron variant (B.1.1.529), which was discovered in November 2021.
Lower hospitalization rate: studies and observations have shown that the Omikron variant leads to a lower likelihood of severe disease progression and therefore a lower hospitalization rate compared to previous variants such as Delta. Estimates from various countries suggest that the hospitalization rate for Omikron infections is between 1% and 3%, depending on the region and population group.
Question for Chat GPT:
“What percentage of COVID infections with Omicron result in Long COVID?”
Answer Chat GPT:
“The likelihood of developing Long COVID after COVID-19 infection with the Omicron variant is the subject of ongoing research, as Long COVID is a complex and not yet fully understood phenomenon.
Here are some key points based on previous studies and findings:
Question to Chat GPT:
“What percentage of COVID infections with Omicron result in death?
Answer Chat GPT:
“The case fatality rate (CFR) for COVID-19 infections varies by variant, vaccination status, age, health status and other factors. The Omikron variant (B.1.1.529) has been shown to be associated with a significantly lower mortality rate compared to previous variants such as Alpha or Delta. Here are some relevant points:
Chat GPT chatbot, queried as of 17.08.2024. Link
Sunday, August 11th, 2024
Overview of influenza, COVID and RSV
Certain viruses can cause severe cases. These include the influenza virus (flu), the coronavirus (COVID) and the RSV virus (respiratory syncytial virus).
References
1 ARE weekly report of the RKI, week 31/2024, link
Saturday, March 5th, 2022
Protective effect of a booster
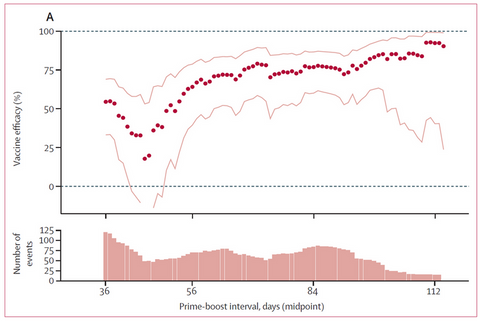
The first diagram shows the remaining protective effect after dose 2 (circles: omicron, squares: delta), after a booster with Biontech/Pfizer („BNT162b2“) and after a booster with Moderna („mRNA-1273“).
The protection against omicron diminishes steeply after only 2 doses (see circles). A booster provides a persistent protection against delta while this effect decreases against omicron after 10 weeks.
This proves why a second booster is necessary in certain groups like individuals above 70 years of age (see our previous article).
The second diagram2 (data of the RKI) shows hospitalisations depending on age and vaccination status.
Individuals without vaccination are hospitalised much more often, independent of their age. After three vaccinations hospitalisation is less frequent than with two vaccinations.
The third diagram2 shows that omicron is dominant in Germany by now.
Conclusion:
At least three vaccinations are recommended for all individuals above 12 years of age. Individuals above 70 years of age should receive a fourth vaccination.
References
1 Andrews N et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. New England Journal of Medicine, March 2, 2022. Link
2 Wochenbericht des RKI zu COVID-19 vom 03.03.2022. Link

Saturday, February 19th 2022Second booster and "mild" COVID
Why is a (first) booster necessary1?
Two vaccinations yield a protection of only 10% against an Omicron infection. Giving a booster improves your protection to 65-75%.
Vaccine efficacy against hospitalisation due to COVID is 89% after the booster. Thus, protection from a severe course in Omicron infections is still very good.
Moderna (Spikevax®) is slightly more efficient than Biontech (Comirnaty®) in it’s function as a booster.
Individuals after the booster infected other individuals in 30% less cases than those with two vaccinations.
Not vaccinated individuals infected 40% more cases than those with two vaccinations.
Why a second booster against Omicron1?
Vaccine efficacy against an infection with the Delta virus was 95.2% after the first booster. Protection against the Omicron variant was only 62.5%, in individuals with a weakened immune system even only 11.5%.
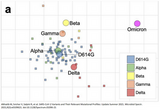
The reason for this is the number of mutations in the Omicron variant. Therefore, Omicron differs a lot from the other viruses like Alpha or Delta (see second image). Antibodies after vaccination do not fit to the Omicron variant as well as to other viruses. They can’t bind and neutralize viruses as they did with Alpha or Delta.
Israeli data in 550.000 individuals with a second booster show a reduction of COVID infections by half and four times less severe COVID infections. This proves the benefit from a second booster.
In Israel, the second booster was administered in individuals above 60 years of age and four months after the first booster. According to preliminary information, side effects were mild and similar to the first booster.
Individuals with three vaccinations and a COVID infection afterwards will not need a second boosters until further notice.
How dangerous is Omicron1?
In comparison to the Delta variant, the risk of hospitalisation with the Omicron variant was 40-80% lower. 0.9% of all infections in Germany until January 20th were hospitalized, 0.05% deceased.
Thus, the course of a COVID infection with Omicron is milder than with Delta.
Nevertheless, a simple calculation shows that Omicron should not be underestimated: The maximum of the Delta wave had 75.000 infections daily while the Omicron wave had 250.000.
Thus, even given a milder course of Omicron there might be twice as many hospitalisations and 1.6 times more deaths after the Omicron wave maximum than after the Delta wave maximum. And this does not consider that medical institutions might be out of capacities.
What exactly is “mild” COVID2?
We should not underestimate a COVID infection. A mild course does not mean that we only get a "running nose”.
If you suffer from fever above 102°F, strong myalgia, headaches, exhaustion and a loss of taste and smell and spend the whole week in bed, you have “only” had a mild course.
A moderate course includes pneumonia. A severe course is defined by a significant drop in oxygen levels (sO2 < 94%) or a respiratory rate above 30 per minute.
A critical course leads to respiratory failure (ventilation necessary), septic shock (e.g. adrenalin administered on intensive care) and/or other organ failures like kidney failure (e.g. dialysis necessary).
Summary
A first and in some cases second booster is necessary for an efficient protection from COVID since the Omicron variant is quite different from preceding coronaviruses.
Omicron seems to be milder than Delta, nevertheless more hospitalisations and deaths are possible due to the extremely high number of infections.
A “mild” course of COVID can also mean to spend a week in bed, feeling really sick and completely exhausted.
References
1. Recommendation of the STIKO, Epidemiologisches Bulletin 7/2022, February 17th 2022. Link
2. NIH COVID-19 Treatment Guidelines, Clinical Spectrum of SARS-CoV-2 Infection, Last Updated: October 19, 2021. Link

Saturday, February 5th 2022Second booster, Omicron wave and new drugs
On February 3rd 2022, the German Vaccination Commission (STIKO) recommened1 a second booster for individuals above 70 years of age and for individuals with immunosuppression. The second booster is due three months after the first. This is thought necessary because of the quickly waning immunity against the omicron variant. Details about this recommendation will follow shortly so an individual decision can be made.
Become infected with omicron and get over with COVID? Not really.
In Europe several omicron strains are found (also BA.2). Therefore, several infections within a short time (weeks) are possible. Moreover, an infection with omicron won’t yield a (sufficient) protection against other variants (delta, beta etc.) since those are too different from omicron. More details in a podcast2 with Prof. Drosten.
Young and healthy individuals shouldn’t feel too safe with a COVID infection if they are not vaccinated. They might suffer from a long COVID syndrome that will render a normal life impossible for weeks or even months (see our article). A study from Israel3 shows that a COVID vaccination reduces the risk of long COVID close to zero.
Novel drugs for the early phase of a COVID infection
Paxlovid® (Nirmatrelvir)4 seems to be highly efficient and is taken orally. In a press release a 89% reduction of mortality was reported. That would be more than impressive. Details will follow.
Molnupiravir5 is an already available drug that is taken orally. It reduced hospitalisation and mortality by 53%. It has been successfully used once in our practice.
Sotrovimab6 is an antibody against SARS-CoV-2. It is applied i.v. and reduced hospitalisation and mortality by 85%. Most of the other available antibodies were rendered ineffective by the omicron variant.
References
1. Statement of the German Vaccination Commision (STIKO) on Feb 03, 2022. Link
2. Coronavirus-Update podcast of the NDR on Feb 01, 2022: Link
3. Kuodi P et al: Association between vaccination status and reported incidence of post-acute
COVID-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. medRxiv preprint. https://doi.org/10.1101/2022.01.05.22268800. Link
4. Mahase E: Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ2021;375:n2713. Link
5. Bernal AJ et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. December 16, 2021. N Engl J Med 2021; online ahead of print. Link
6. Gupta A et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. November 18, 2021. N Engl J Med 2021; 385:1941-1950. Link

Wednesday, January 5th 2022Preliminary information on the omicron variant
Mutations, vaccine efficacy and infectiosity 1,2,3
The diagram on the left shows the original Virus in the first row (Wuhan virus), the delta variant in the second and the omicron variant in the third row. The first column shows the number of mutations, the second the vaccine efficacy and the third the infectiosity of the virus variant.
Results are:
- Vaccine efficacy against the original virus is almost 100% with 2 or 3 doses.
- Due to mutations (delta has 9, omicron has 34 mutations), vaccine efficacy against delta with two doses went slightly down while efficacy against omicron is strongly reduced. With 3 doses, vaccine efficacy against delta and omicron is sufficiently high.
- The last column shows that delta is more infectious than the original virus (about twice as infectious) while omicron is more infectious than delta (again about twice as infectious).
Actual data seem to show that the omicron variant leads to less hospitalisations than delta (approx. 30-60% depending on source).
This extremely high wave of infections endangers staff in key positions like law enforcement and hospitals. Capacities will be reduced, including situations with health issues that are not correlated with COVID. Since patients will more frequently seek assistance in practices and at general practitioners, there will also be capacity issues there.
Reduction of contacts and hygiene measures
The number of patients without adequate access to hospital or a physician can only be kept low by flattening the wave. Thus, less patients will be infected with COVID at the same time. Everyone should reduce contacts to a minimum and carry masks properly, especially covering the nose to avoid spreading aerosols.
Omicron: back to start? No.
It took almost two years for a virus variant to appear that partly compromises vaccine efficacy. Therefore, there should be enough time to adapt vaccines to the omicron variant (if at all necessary, under investigation). This process takes 3-6 months with mRNA vaccines and about 1-2 years with protein vaccines (e.g. "Novavax").
At the moment, around half of the world’s population has received at least one vaccine dose. This is the right and only way out of the pandemic and should be followed through. After vaccinating as many people as possible, the virus will find less and less places to spread and develop mutations.
Depending on how well actual mRNA vaccines work against omicron (data pending), a vaccination with a new vaccine adapted to omicron might be necessary in spring or early summer. If it proves unnecessary to adapt the vaccine, we should prepare for probable vaccinations in winter 2022 and 2023.
At the moment, everyone should get the third COVID vaccine dose as quickly as possible to be protected against the omicron variant.
References
(1) Garcia-Beltran WF et al, 2021, preprint: mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. (link)
(2) Collie S et al, 2021: Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. (link)
(3) Nemet I et al, 2021: Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. (link)
(4) UK Health security agency, technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC-21 NOV-01 (B.1.1.529). (link)
(5) COVID Dashboard of the Johns Hopkins university, updated daily (link)
Sunday, November 21st 2021Less Biontech/Pfizer available – what does that mean?
What does this change mean?
More time involved for our staff
On the one hand, organizing vaccination appointments will be more complex: Not only 7 patients have to fit into one vaccination appointment (1 vial of Biontech is for 7 people), but even 20 patients (1 vial of Moderna is for 20 boosters). On the other hand, especially German patients will ask more questions, and that will take more time.
Moderna and Biontech equivalent for most patients with slight advantages in favor of Moderna
There are hints in studies that Moderna boosters yield a better protection against COVID breakthrough infections than Biontech1.
The diagram on the left shows: The upper curve (grey) is the number of infections over time in patients without any vaccination. The middle curve (blue) is the number infections after a booster with Biontech, and it shows much less COVID infections. The lowest curve (orange) represents infections after a booster with Moderna, and that one has the least number of COVID infections.
However, in young individuals there are fewer side effects in the sense of myocarditis with Biontech than with Moderna. Therefore, the German Vaccination Commission (STIKO) has recommended to vaccinate indidivuals below 30 years of age only with Biontech2. Out practice will comply with this guideline.
Conclusion
Altogether, it is crucial to have a booster at all with an mRNA vaccine like Moderna or Biontech. Both vaccines are highly efficient against an infection with COVID-19. The benefit is shown in analyses in the USA3 and Canada4. Thus, both vaccines are interchangeable for all patients above 30 years of age with a slight advantage for Moderna.
1. Puranik A et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv [Preprint]. 2021 Aug 9:2021.08.06.21261707. doi: 10.1101/2021.08.06.21261707. PMID: 34401884; PMCID: PMC8366801. Link
2. Epidemiologisches Bulletin 46/2021 des Robert-Koch-Instituts vom 02.11.2021. Link
3. Nanduri S et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1163-1166. doi: 10.15585/mmwr.mm7034e3. PMID: 34437519; PMCID: PMC8389386. Link
4. Chung H et al. Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021 Aug 20;374:n1943. doi: 10.1136/bmj.n1943. PMID: 34417165; PMCID: PMC8377789. Link

Thursday, November 18th 2021
COVID booster for all individuals above 18 years of age
- All individuals above 18 years of age are recommended to receive a booster vaccination.
- Indidivuals above 70 years of age, individuals living in parental homes and medicals staff should be prioritized.
- The booster should be applied 6 months after the last COVID vaccination, in case of sufficient capacities already after 5 months.
- An mRNA vaccine („Biontech“ oder „Moderna“) should be used to booster, independent from the vaccines used beforehand.
All in all we are getting ahead very well: We are already making appointments for individuals above 60 years of age. In order to protect as many people as quickly as possible we also vaccinate on Saturdays and Sundays.

-

Sunday, October 10th 2021
Booster vaccination against COVID
Booster vaccination against COVID

Sunday, September 26th 2021
Booster vaccination currently only for patients with immunodeficiency
Booster vaccination currently only for patients with immunodeficiency
The vaccination commission has recommended a booster dose for patients with an immunodeficiency1.
In case of a mild immunodeficiency, this should happen after six months (e.g. after two doses of Biontech/Pfizer or Moderna), in severe immunodeficiency already after four weeks.
As of now, a simultaneous vaccination with other vaccines, e.g. influenza, is possible.
What about individuals above 60 years of age without immunodeficiency?
There is (still) no general recommendation for a booster dose above a certain age. At the moment there is insufficient data to prove a benefit. This might change in the following weeks.
The vaccination is not meant to protect from any infection but only from severe courses. If a patient has a mild disease instead of a severe one, the vaccination was successful.
Statistics in Germany2 show a COVID infection in vaccinated individuals above 60 years of age in only 5,6% of all COVID cases of this age group. Most important is the number of COVID cases on intensive care (above 60 years of age): 97,2% are not vaccinated, 2,8% are vaccinated.
The diagram opposite3 clearly shows that it is the unvaccinated population that is predominantly hospitalized. Thus, the expression “pandemic of the unvaccinated” is accurate.
Registration for the booster
The following patients can register for a COVID booster in case the following conditions or medication apply (selection, detailed list see table):
Condition:
Organ transplant, stem cell transplant, dialysis, chemotherapy in cancer, rheumatoid arthritis, multiple sclerosis, lupus erythematodes, Crohn’s disease, Ulcerous colitis, HIV infection.
Medication:
Methotrexate, ciclosporin, azathioprine, mycophenolate, systemic cortisone therapy.
If you’re not sure whether you belong to this group, please contact your physician.
Sources:
1 Epidemiologisches Bulletin des Robert-Koch-Instituts, Nr. 39/2021. Link
2 Wochenbericht des Robert-Koch-Instituts vom 23.09.2021. Link
3 Wochenbericht des Robert-Koch-Instituts vom 02.09.2021. Link

Sunday, September 19th, 2021COVID Vaccination in pregnant and breastfeeding women
Since Sept. 16th, 2021, the German Vaccination Commission (STIKO) recommends an mRNA COVID vaccination in pregnant women from the second trimester and in breast-feeding women. It also strongly recommends a vaccination in women of childbearing age to acquire immunity before conception.
Two administrations of Biontech (Comirnaty®) with a 3-6 week interval or Moderna (Spikevax®) with a 4-6 week interval are possible.
The goal is to avoid severe COVID-19, fatalities and complications due to COVID-19 during pregnancy and to the unborn child.
COVID-19 infection in pregnant women: a higher risk
During pregnancy, the number of certain cells of the immune system diminishes (CD4 and CD8 lymphocytes, killer cells). This might be the reason for a higher risk of severe COVID-19 courses in pregnancy.
Lung capacity goes down during pregnany. Thus, pulmonary infections can have a more severe effect.
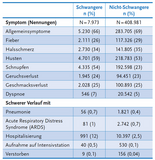
Pregnant women with a COVID-19 infection are hospitalized in every 8th case. This is much more frequent than in non-pregnant women of the same age (see table). Pregnant women are admitted to intensive care five times more often and decease twice as often as non-pregnant women.
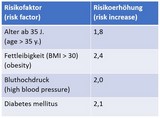
Conditions that increase the risk of severe COVID-19 in pregnancy are: high blood pressure, diabetes, preeclampsia, age and higher weight (see table, a factor of 2 represents a risk twice as high).
COVID vaccination in pregnant and breast-feeding women
Vaccine efficacy in pregnancy is 97% in avoiding symptomatic COVID infections.
Antibodies are transferred to the unborn child. It is possible that this protects also the child from COVID, but this is not yet confirmed by studies. Antibodies are also in the mother’s milk. The child might be protected from COVID by taking in the mother’s milk, but this is also not yet proven.
As of now, no cases of severe vaccine side effects have been observed in pregnant or breast-feeding women and their children.
Conclusion
COVID-19 is much more dangerous to pregnant women than to non-pregnant women of the same age. The available vaccines protects extremely well from severe courses of COVID-19. Side effects of the vaccine are generally mild.
Source: epidemiologic bulletin of the Robert Koch institute Nr. 38, 2021, published online Sept. 16th, 2021

Saturday, August 28th 2021COVID vaccination in children and adolescents 12-17 years of age
The German Vaccination Commission has recommended1 on August 18th 2021 to vaccinate children and adolescents 12 to 17 years of age with one of the mRNA COVID vaccines against COVID-19 (Biontech (Comirnaty®): interval 3-6 weeks, Moderna (Spikevax®): interval 4-6 weeks).
Vaccination side effects1
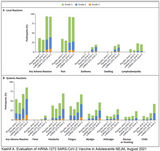
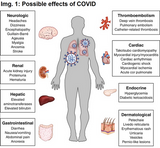
After vaccination children suffered from pain at injection site (91%), fatigue (78%), headache (76%), chills (49%) muscle ache (42%), fever (24%), joint ache (20%), swelling at injection site (9%), redness at injection site (9%), palpable lymph nodes (0,6%). See first image.
Vaccine efficacy 100%1
The COVID vaccines showed an efficacy of 100% in the phase 3 trial.
Myocarditis (inflammation of the heart muscle) after COVID-19 infection and after COVID vaccination
A very seldom side effect of the vaccination is a myocarditis that appears in 1 of 17.000 cases in boys (12-17 years of age) and in 1 of 110.000 cases in girls (12-17 years of age)1.
But COVID-19 also causes a myocarditis, and even more often than the vaccination does: In boys it happened 6 times more often, in girls 23 times more often2 (Singer ME et al).
Thus, the advantages by far outweigh the disadvantages of a COVID vaccination. It is much more risky not to have a COVID vaccination and get the disease (everyone will get the infection in the next 2-3 years, see my last article). See second image: expected next wave in Germany according to Robert-Koch-Institut1.
PIMS in children due to COVID-19: 71% on intensive care, 1,7% do not survive
COVID-19 can lead to PIMS3 (pediatric inflammatory multisystem syndrome) in children (Nikolopoulou et al). Estimations are that one out of 2400 COVID cases in children will suffer from PIMS.
39 studies with 662 children showed that PIMS leads to fever and in 60% to a state of shock. 71% of cases need intensive care, 22% need ventilation. 1,7% decease. The long-term effects of PIMS are unknown.
Long-COVID in children: one out of 20 children are sick for more than 4 weeks
Children with Long-COVID suffer from shortness of breath, cough, energy drain, sleep disturbances, arrhythmias and more. A British study with 1734 children shows that one out of 20 children were sick for at least 4 weeks, one out of 50 for at least 8 weeks4 (Ahmed M et al).
Conclusion: COVID-19 is dangerous also for children
The majority of children have a mild COVID-19 disease or even no symptoms at all. However, severe courses happen, and these are much more often than any known or unknown side effects of the vaccine.
1 Robert-Koch-Institut, Epidemiologisches Bulletin Nr. 33 vom 19.8.2021
2 Singer ME et al. Risk of Myocarditis from COVID-19 Infection in People Under Age 20 A Population-Based Analysis. Preprint, medRxiv, July 2021.
3 Nikolopoulou et al. COVID-19 in children: where do we stand? Arch Med Res., July 2021
4 Ahmed M et al. Multisystem inflammatory syndrome in children: A systematic review. Lancet, September 2020.
5 Molteni E et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. The Lancet Child & Adolescent Health. August 2021.

Monday, August 9th 2021COVID vaccination: yes or no? How to reach a decision.
1. Is it possible to permanently avoid an infection with the coronavirus?
2. What is worse: COVID-19 or the vaccine?
3. Does the vaccine protect me efficiently from COVID-19?
Short answers:
1. Every single person in the US, Europe and other densely populated areas around the world will get infected with the coronavirus within the next 1-2 years (Torjesen I).
2. In young people, the most important side effect of a vaccination is a myocarditis (inflammation of the heart muscle). But the coronavirus also causes a myocarditis, and this happens even 40 to 2000 times more often than due to a vaccination. This is the result of a current CDC analysis of 50 million vaccinated young individuals 12-29 years of age: 1 million vaccinations with Biontech/Moderna caused 5 cases of myocarditis, but also saved 6 young lives, avoided 73 intensive care admission, 922 admissions to hospital and avoided 12.500 infections with COVID-19 (Gargano JW et al).
3. The vaccination (Biontech) protects from any infection (also light cases) in the alpha variant in 95%, from the delta variant in 88% (Lopez Bernal J et al). The protection from severe course is 100% in the alpha variant. In the delta variant it should be similarly high.
COVID vaccination: yes or no? A detailed answer.
No one in the US or Europe will escape the coronavirus within the next 1-2 years (Torjesen I). No matter how seldom you meet other people. And mask mandates will (luckily) not be permanent. Prof. Christian Drosten (virologist from Berlin University) (Source):
“This virus will become endemic; it will not go away. Whoever actively decides against a vaccination will unavoidably get infected. You can’t do anything about it.”
“100%, not 70 or 80, but 100% of the population will unavoidably become immune within a time frame of more or less one and a half years. Either through vaccionation or by natural infection.”
Let me add: Becoming immune through natural infection can be accompanied by long-term harms to your health. More about that in the next chapter.
What is worse: COVID-19 or the vaccine?
Health risk caused by COVID-19
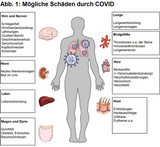
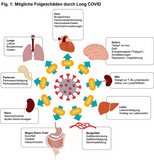
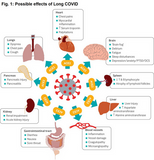
On image 1 (Gupta A et al) you can find diseases caused by COVID. Among these are: pneumonia, pulmonal embolism, thrombosis, heart attack, stroke and other severe diseases. More about long COVID down below.
20 % of COVID cases lead to admissions to hospital. A fifth of those has a severe course, i.e. needs invasive ventilation or doesn’t survive (Bennett TD et al).
In-patients suffer from a thrombosis in approximately 1 of 14 cases and from a pulmonal embolism in 1 of 10 cases (Suh YJ et al). More precise data will follow.
COVID causes myocarditis (inflammation of the heart muscle). A study with competitive sportspersons showed an incidence of 1 myocarditis in 500 COVID cases (Daniels CJ et al).
The risk of heart attacks and strokes is increased by 290% (Mafham M).
This is just a selection of many health risks caused by COVID. Since COVID is a new disease, further consequences of an infection might still be discovered in the future.
Long-COVID describes patients in which symptoms due to a coronavirus infection persist for more than 4-12 weeks.
Long COVID affects 10-30% of patients (> 4 weeks in 30%, > 12 weeks in 10%) (Alwan NA).
Symptoms can be shortness of breath, palpitations, energy drain, fatigue, persisting loss of smell and taste, issues with concentration, sleep disturbances and other, see fig. 1 (detailed description by Crook H et al).
Young individuals will not be spared. Even now in Great Britain, 2 in 1000 children in the age of 2-11 years and 5 of 1000 adolescents in the age of 12-16 years suffer from long COVID. Please note: Those 1000 are all children or adolescents, not only the COVID cases (Crook H et al).
After 2 years of pandemic, we have to expect approximately 75.000 children and 55.000 adolescents with long COVID in the US if they will get infected with the coronavirus without the protection of a vaccination.
Side effects of the COVID vaccine (only Biontech/Pfizer)
Just for a moment, please forget about the misleading "information” that is forced on you on social media and in the internet.
The current COVID vaccines are being observed very closely in the US (CDC), in Europe (eCDC) and Germany (Paul-Ehrlich-Institut). Every suspicion of a side effect is being tracked attentively and thoroughly.
When a whole nation gets vaccinated, it is only natural that events will be observed with or without a vaccination. The incidence of health events is an everyday matter for me and might be forgotten about when you don’t have to do with medicine every day. However, the crucial point is to distinguish between an event that is due to a vaccination and an event that would anyway have happened.
On closer examination, two side effects of the Biontech/Pfizer vaccine should be looked at because of their significance and frequency: myocarditis (inflammation of the heart muscle) and anaphylactic allergic reactions.
The incidence of a myocarditis strongly depends on the age of the patient. Adolescents 12-17 years of age have the highest incidence with 1 myocarditis in 20.000 vaccinations. When you look at all age groups, the incidence is only 1 in a million. (Safety report of the Paul-Ehrlich-Institute, June 30th 2021)
Anaphylactic allergic reactions happened once in 300.000 vaccinations (201 cases in 54.898.640 vaccinations with Biontech/Pfizer, data of the PEI, see above).
Comparison between COVID and vaccination
The previous information helps us to understand that COVID-19 is a dangerous disease. The harm to our health is much greater and more frequent than any side effect by vaccination.
The closest thing to a direct comparison is looking at myocarditis: Due to vaccination, one myocarditis in 20.000 adolescents was detected (one in a million in people of any age).
But: In competitive sportspersons, a COVID infection caused a myocarditis in 1 of 500 cases [Daniels CJ et al).
Thus, a myocarditis caused by COVID was observed 40 to 2000 times more often than due to a vaccination.
The benefit of a vaccination is proven in an analysis of the CDC: 52 million adolescents and young individuals 12-29 years of age have been vaccinated against COVID in the US (Gargano JW et al): In one million vaccinations, 5 cases of myocarditis have been observed. At the same time, 6 deaths (of young people!) have been avoided, also 73 admissions to intensive care and 922 admissions to hospital (see table 2).
Conclusion: Even if we look at the most significant potential side effect of a vaccination in young individuals, COVID-19 is still incomparably more dangerous than the vaccine. Ergo, it makes sense to get vaccinated.
Misunderstandings concerning the COVID vaccine
The first impression stays – even if it is wrong
Unfortunately, our brain works in a way that the first impression cannot be easily influenced or corrected by information that comes afterwards.
There are people who take advantage of this effect: They spread outlandish theories and disinformation without supporting it with solid proves but instead inciting fears with emotionally charged messages.
It is much easier to spread nonsense than to practice proper science. Vince Ebert, a German satirist and physicist, said: “That’s why an esoteric can spread more nonsense in five minutes than a scientist can disprove in his whole life.” (Spektrum)
The scientific method needs time, but it is the only reliable way to prove a claim. Again Vince Ebert:
“Simply said, the scientific method is a way to test a hypothesis. If I believe: “There’s beer in the fridge”, and I go and check, it is an early form of science. However, in theology claims will not be tested. If I only claim [and don’t check]: “There’s beer in the fridge”, I’m a theologist. If I check and look, but I don’t find any beer and still claim: “There is beer in the fridge!”, then I am an esoteric."
Thanks to this knowledge it will be easier for us to correct a first (wrong) impression and substitute it with proven information. And that’s of crucial importance in a pandemic since it will protect our health, possibly our life, but certainly the life of friends or family members with risk conditions.
Commentary on other rumours
Fertility
There is nothing pointing to a connection between fertility and vaccination. Therefore, claims regarding this topic are at the same time suprising (for physicians), and not surprising at all (for psychologists): Fertility is such an emotionally charged topic that it sends our reason off on holiday. Thus, logical arguments are not of importance anymore.
The fact is: If there were a risk, it would already have been detected. But there is none. The safety report of the Paul-Ehrlich-Institute in Germany hasn't found anything, and they look really very closely.
Background information: Absurd claims say that the spike protein (appears after vaccination) has similarities to syncytine-1 that they say is important for fertility. But there is no similarity. And if it were like that, all those people infected with COVID (myriads of spike proteins invading the body) would have to suffer from consequences, too, which is not the case.
In COVID-19 infection the number of spike proteins (including genitals) invading the body is incomparably higher than the minuscule quantitiy of spike protein administered to one’s arm only (staying in the arm and dissolving after some days). Moreover, in a real COVID-19 infection, one’s body is attacked by a dangerous, live virus.
More about that topic can be found in the coronavirus update podcast with professor Sandra Ciesek (virologist Frankfurt university) from minute 65, reading version here.
Genetic modification
The Biontech/Pfizer vaccine is based on mRNA, a simple construction plan for proteins. It has nothing to do with the cell’s DNA, the mRNA doesn’t even have access to the cell’s nucleus, the place where the DNA is located.
The mRNA just fulfils its purpose: It leads to the production of spike protein to train our immune system. This way, our body can provide antibodies and other means of defence for a real infection.
However, the coronavirus invades the cell’s nucleus, it can change the cell’s DNA and “enslaves” the cell: It stops normal cell functions and forces it to do everything to promote its proliferation (Scudellari M).
By the way: Genes of muscle cells in one’s arm (the place the vaccine is injected into) don’t have anything to do with the genes of our germ cells, i.e. sperm cells and ova. The vaccine doesn’t get to the genitals, but the coronavirus goes everywhere.
Long-term effects of the vaccine and the coronavirus infection
Side effects of a vaccination are to be expected in the first days and weeks, but not longer than three months. It is extremely improbable to observe anything later-on. This is what is known from other vaccines.
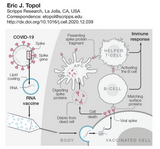
If you look at the mechanism of the COVID vaccination, it becomes clear why long-term effects are not to be expected. More on that on the diagram by Eric Topol and in this detailed review on vaccines in general (Pollard AJ et al).
However, in a coronavirus infection long-term side effects are definitely to be expected. Those can be due to consequences of diseases caused by COVID (e.g. pneumonia, heart attack) and also by long COVID. And since COVID is a novel disease, we probably still don’t know about all possible harms to our health.
Therefore, we should focus more on long-term consequences due to a coronavirus infection since they are proven and frequent. In contrast to that, the vaccine has proven to be safe after 14 months of observation (most recent data on the Biontech/Pfizer vaccine).
Here are the numbers from my practice: approximately 1300 vaccinations, no severe side effects. In contrast to that: 100 COVID infections, among those 2 patients deceased, 15 cases of long COVID, predominantly among young patients.
Accelerated vaccine development
See my article.
Corona tests also work after a vaccination
Rapid tests and PCR tests work properly after a vaccination. Rapid tests detect protein fragments while PCR tests detect DNA fragments. The protein fragments (spike protein) that appear after vaccination remain in the arm and dissolve after some days. They don't interfere with a swab test.
COVID-19 is real and leads to numerous casualties
The diagram (EuroMOMO) shows the effect of the Corona pandemic on mortality rates in Europe.
The grey zone is the expected mortality, crossing the red line would show an excess mortality. As shown on the diagram, in spring 2020 and autumn 2020 / winter 2021 mortality rates were way out of range. Hence, the association between the corona pandemic and the rise in fatalities is obvious: COVID-19 has already cost us many lives.

Friday, July 9th 2021
Heterologous vaccination with Biontech after AstraZeneca: a tactical decision.
Heterologous vaccination with Biontech after AstraZeneca: a tactical decision.
last week the German commission for vaccinations (“STIKO”) has surprisingly recommended vaccinating all individuals with Biontech as the second vaccine if those individuals had AstraZeneca as their first (“heterologous” vaccination) (original publication).
Now the scientific reasons for that recommendation have (finally) been published:
1. Recent studies seem to show that a heterologous vaccination leads to higher antibody levels than a vaccination with AstraZeneca only (as first and second vaccine).
2. A single vaccination, no matter with which vaccine, yields a much less effective protection from the delta variant of SARS-CoV-2. Therefore, an early full vaccination is needed. With a heterologous vaccination, a full vaccination can be reached faster: The interval between the shots can be reduced from 12 to 4 weeks. It seems that a reduction of the time interval does not diminish vaccine efficacy.
3. “A vaccination with AstraZeneca as the second vaccine is acceptable if the appointment for the second vaccination with AstraZeneca is due soon. A homologue vaccination with AstraZeneca also protects very well from a severe infection with the delta variant of SARS-CoV-2.”
Discussion:
1. It’s important to mention that the studies on which these recommendations are based on are very small: The most important study (Shaw et al.) includes only 830 individuals that are divided into different study groups. Thus, for the assessment of the combination of Biontech 28 days after AstraZeneca only approximately 100 individuals were included. In comparison to that, the original studies with the conservative vaccination scheme included 24.000 (AstraZeneca) and 38.000 (Biontech) individuals. These large scale trials didn’t only address antibody levels but also the crucial question whether the vaccination protects from an infection with SARS-CoV-2.
2. Therefore, the STIKO recommendation concedes: “Lab results suggest a stronger immune response but it is unknown whether this will yield a better protection from an infection with SARS-CoV-2.”
3. It seems probable but it is not yet certain that a shortened time interval between vaccinations (Biontech after AstraZeneca) will lead to the same vaccination efficacy.
4. There are no long-term data about how long the protection of a heterologous vaccination will last.
5. The STIKO’s recommendation is surprising since it usually gives quite conservative opinions, e.g. there is still no green lights to vaccinating pregnant women and children. In other countries a heterologous vaccination is not yet recommended while pregnant women and children are already being vaccinated.
Conclusion:
Regarding actual data, a heterologous vaccination with Biontech after AstraZeneca seems a reasonable strategy except for the above-mentioned precautions. Therefore, I would rather support the STIKO’s recommendation. The (informed) patient’s decision is of critical importance here.
At the same time, I would like to stress that the most important decision is whether to get vaccinated at all. In comparison to that, the decision about a heterologous vaccination is only a detail with potential advantages that one can use but doesn’t have to.
Saturday, April 24th 2021
AstraZeneca: My last comment.
AstraZeneca: My last comment.
I regret that unbalanced reports in the media have led to confusion and even alarm regarding the AstraZeneca vaccine. And I understand these concerns very well: All the time we hear unsettling reports without the comments that will put everything into a context, mentioning the advantages or comparing the vaccine with the risks of other vaccines or the risks of everyday life.
Reporting of this kind will make people get vaccinated much later, possibly millions. This implies that part of those people will get infected with COVID, suffer from Long-Covid in 10% and die from COVID in 1-2%. Especially in a pandemic unbalanced information damages our health and even costs lives.
This is why I’d like to compare advantages and disadvantages of the AstraZeneca vaccine for a last time. Cambridge University has published an instructive diagram (see first diagram):
The dots on the left side show how many admissions to intensive care could be avoided by an AstraZeneca vaccination (in 100.000 individuals).
The dots on the rights side show how many severe adverse events were reported in connection with the vaccination (in 100.000 individuals).
Even in a situation with less infections (low incidence), the first diagram shows that indivuals above 60 years of age have a benefit from the vaccine that is 70 times higher than any severe adverse effects. It is only in individuals below 30 years of age that the potential damage might outweigh the benefits: This is why AstraZeneca will not be administered to individuals below 30 years of age, but is approved in all others above 30 years of age.
In a situation with many infections (high incidence in a "wave" like we have it now), the difference becomes huge: In individuals above 60 years of age, the benefit of a vaccination is 600 times higher than any severe adverse event. The vaccine saves 600 people from becoming critically ill and go to intensive care while risking a severe adverse event in one single individual.
In other words: If a physician decided not to vaccinate people above 60 years of age with AstraZeneca, he might save a single patient from a severe adverse event but at the same time cause 600 other patients to become critically ill from COVID and need intensive care. A high percentage of those critical COVID cases would die. Probably all survivors will suffer from a permanent damage. Let’s ask ourselves: Would it really be wise and ethical not to vaccinate with AstraZeneca?
Please let us remember that the other vaccines are not flawless, either. Above a certain age, but certainly above 60 years of age, there is no relevant difference in risks between all approved vaccines. These are the important things on which we should focus and base our decisions on.
The virus doesn’t care if we are afraid of side effects of a vaccine: It will continue to chase us. And only a vaccine can save us from an infection and especially from a severe course of the disease. Thus, one can only lose by rejecting a vaccine that would save millions.

Sunday, April 18th 2021
COVID-19 vaccines and their side effects: the facts.
COVID-19 vaccines and their side effects: the facts.
according to media reports, "one" vaccine is dangerous, the "other" is flawless. That's not correct. Here's my clarification, based on the safety report of the Paul Ehrlich Institut (PEI) in Germany. The PEI is responsible for the supervision of the safety of vaccines.
Venous sinus thrombosis
In individuals above 60 years of age, 5 cases of venous sinus thrombosis have been observed after Biontech/Pfizer vaccination and 2 cases of venous sinus thrombosis after AstraZeneca vaccination. Considering the number of vaccinations (see slide 1), the resulting incidence is very similar in both vaccines and amounts to approximately 1 : 1.000.000.

Wednesday, March 31st 2021
AstraZeneca: What is going on?
AstraZeneca: What is going on?
I understand the confusion and uneasiness about the AstraZeneca vaccine. This article will give some answers.
Why had the vaccine been limited to younger patients in January?
At that time there was too little data of individuals older than 65 years. Thus, data was not sufficient to assess the efficacy. This resulted in approving the vaccine for younger patients only.
Why do we find different numbers regarding efficacy?
We are experiencing science live. It is normal to have new data all the time that is analysed and leads to new conclusions. Unfortunately, the media gives us an exaggerated and shrill image of the situation, and that’s not good. On the other hand, the efficacy of the AstraZeneca vaccine is very good (see my article below).
What about the accumulation of venous sinus thrombosis?
When millions of people are observed, many cases of illness and deaths will happen. This is part of everyday life. But the crucial question is whether there is a causal connection to the vaccine or not.
Germany believes that the accumulation of cases is significant enough to limit the approvement of the vaccine to individuals above 60 years until the question of causality is examinated more closely.
However, England observes much less cases of venous sinus thrombosis. Therefore, the AstraZeneca vaccine is still approved for individuals of any age.
Does the vaccine cause a venous sinus thrombosis?
This is unknown and being examinated. There is a preliminary explanation that presumes a pseudo-allergic reaction in which antibodies activate platelets to form a thrombosis (Greinacher et al).
It is also unknown which part of the vaccine causes this “allergy”. It might be a part of the vaccine’s “transporter” (vector). In that case, not only AstraZeneca would cause problems, but also other vector vaccines like “Sputnik” and “Johnson & Johnson”.
If it is the spike protein to cause the above-mentioned reaction, all approved vaccines might be affected. However, in case of a coronavirus infection much more quantities of spike proteins are produces in the whole body and not only in the upper arm.
What is the risk of thrombosis?
Until present time, 31 cases of venous sinus thrombosis have been observed among 2,8 million vaccinees (AstraZeneca) in Germany (RKI statistics).
Thus, we have 1 case among 90.000 vaccinees.
On the other hand, we have 76.000 deaths by COVID among 2,8 million infections in Germany.
Thus, we have 2443 deaths by COVID among 90.000 infections.
Consequently, the risk of death by COVID is 2443 times higher than the risk of venous sinus thrombosis. The older the patient, the higher the risk due to COVID.
Therefore, COVID is incomparably more dangerous, and the pandemic isn’t over yet. Anyone who isn’t vaccinated runs the risk of infections and die from COVID or, much more frequent, suffer from persistent health damage (long COVID in up to 10% of cases, review article)
What to do? Wait for a different vaccine?
For younger individuals, other vaccines do exist. However, it remains unknown whether other vaccines also pose a certain (very low) risk that is still unknown at present time.
If there were only one vaccine in the world, AstraZeneca, the answer would be very easy: Get vaccinated! Corona is much more dangerous than any risk of vaccination. But as long as other vaccines exist, the question remains: Wait for a different vaccine? But waiting would mean to expose yourself to a coronavirus infection. And that would be more dangerous, especially in older patients. For this reason, the AstraZeneca vaccine is currently recommended for individuals above 60 years of age.
Conclusion
Let us wait for the scientific results of the coming weeks. Until then, AstraZeneca remains on halt.
I strongly recommend not to draw any premature conclusions and declare a good vaccine as not suited or even dangerous without having sufficient data.
The best thing to do is to remain calm and objective. Please don’t follow the media to extreme positions.
I will continue to report.

Saturday, March 27th 2021
Corona Vaccinations in our practice
Corona Vaccinations in our practice
we will probably start with vaccinations against Corona on April 7th.
You don’t need to call us: We will call you and make an appointment. Currently we will only vaccinate patients above 80 years of age.
Order of vaccination:
We will prioritize for the risk to suffer from a severe, life-threatening COVID. Above the age of 60 years, age is the primary criterion for prioritization, analogous to recommendations of the Robert-Koch-Institute, Epidemiologic Bulletin, Nr. 2, 2021: „Therefore we assume that the risk of severe or life-threatening COVID for individuals younger than 60 years of age is lower than it is for older individuals even if younger individuals suffer from chronic conditions, no matter if older individuals have a chronic condition or not.”
Second criterion for prioritization are chronic conditions. According to the RKI (see table, same source) those conditions are assigned to risk increase factors that will be included in our calculation of the over-all risk.
This procedure will make sure that those at highest risk will be vaccinated first.
Individuals 60-80 years of age are invited to contact us by e-mail: corona(at)praxis-dr-lahdo.de.
Please tell us: Have you already been vaccinated or do you already have an appointment at a vaccination centre? If not, would you like to have a vaccination in our practice?
We courteously ask patients under 60 years of age to wait until further notice.
In case of questions please exclusively use the above-mentioned e-mail and not the phone because that might block sick patients from reaching us.
We will only vaccinate patients from our practice. We advise other patients to reach out to their personal general practitioner or vaccination centre.

Thursday, March 18th 2021EMA: Benefit of AstraZeneca vaccine outweighs possible side effects
the European Medicines Agency (EMA) has published a recommendation regarding the AstraZeneca vaccine:
- The vaccine does not increase the risk for thrombotic events in general.
- It is possible that there is a connection between the vaccine and a very rare thrombosis of cerebral veins.

Monday, March 15th 2021Commentary on suspected irregularities with the AstraZeneca vaccine
there is some irritation and confusion about the AstraZeneca vaccine in the media. This is my summary of the current state.
The Paul-Ehrlich-Institut (PEI) is responsible for the surveillance of the safety of vaccines in Germany .
The PEI commented today: A thrombosis of cerebral veins with low platelet count has been observed in association with the administration of the AstraZeneca vaccine. It is recommended to consult a physician if a vaccinee suffers from strong and persisting headaches for more than four days or from point-shaped skin bleeding.
It is not yet possible to say whether there is a causal connection to the vaccination. However, it seems rather improbable.
The described events are very seldom. The PEI wrote four days ago: 30 cases of thromboembolic events among 50 millions vaccinations with AstraZeneca are not more frequent than the number of such events among the normal population without a vaccination.
There are irregularities, but their consequences are not yet clear. Therefore, staying alert is adequate. Nevertheless, these events are very seldom, and we all will be relieved if these concerns will vanish into thin air.

Sunday, March 14th 2021
Vaccine Johnson & Johnson "Ad26.CoV.S"
Vaccine Johnson & Johnson "Ad26.CoV.S"
Dear patients,
“Ad26.CoV.S“, another vaccine, has been approved in the US and EU. (CDC Weekly, March 5th 2021).
Summary:
This is another vector vaccine just like the one from AstraZeneca. An adenovirus works as a carrier that is “programmed” with the spike protein of the coronavirus.
Data of 2.650 of 43.783 participants have been analysed. In the placebo group 50 COVID cases have been observed while it was 18 in the verum (true vaccine) group, resulting in an efficacy of 66%. The efficacy in severe cases of COVID was 82%, hospitalisations were avoided in 100%.
Fever as a vaccination reaction appeared in 9% of vaccinees. Side effects were minimal and comparable to those of other vaccines. Severe side effects happened in 0,4%, equally in the placebo and verum group.
Conclusion:
The analysis is not yet complete, there is no scientific publication of the phase III results. Nevertheless, the data justifies the approvement of the vaccine in the USA (FDA) and in Europe (EMA). One important advantage of this vaccine is that it has to be administered only once.
With every additionally approved vaccine we come closer to vaccinating enough individuals to go back to normal life again.

Thursday, February 25th 2021
Vaccine Efficacy of ChAdOx1 (AstraZeneca) depends on interval length between first and booster dose
Vaccine Efficacy of ChAdOx1 (AstraZeneca) depends on interval length between first and booster dose
important new results about the above-mentioned vaccine have been published (Publication Lancet, 19.02.2021).
Summary:
Data of 17178 test individuals have been analysed. 8597 of those have been vaccinated with ChAdOx1. Not a single vaccinee has been hospitalized due to a COVID-19 infection.
A vaccination interval of 12 weeks between the first and second dose resulted in a better efficacy of 81% in comparison to 55% with an interval of < 6 weeks (full standard doses).
The diagram shows that the vaccine efficacy (y axis) increases with the length of the time interval (x axis) between doses (red dots).
Conclusion:
The vaccine ChAdOx1 of AstraZeneca efficiently protects from severe courses of a COVID-19 infection. Vaccine efficacy with a vaccination interval of 12 weeks reaches a very good result of 81%. Therefore, the vaccine is recommended to all individuals 18-55 years of age, provided that the vaccination interval is 12 weeks.
Additional comment:
A vaccine against COVID-19 is not meant to protect from a running nose due to corona, but from a severe COVID-19 infection. Thus, the difference in efficacy between ChAdOx1 and the mRNA vaccines of Biontech/Pfizer and Moderna is only relevant for special situations.

Saturday, February 6th 2021
Coronavirus Vaccine Trial of the Gamaleya Institute, Vaccine „Sputnik V“
Coronavirus Vaccine Trial of the Gamaleya Institute, Vaccine „Sputnik V“
an interim analysis of the phase III trial of “Sputnik V” has been published on February 2nd 2021 (original publication).
Summary
- Data of 19.866 individuals have been analysed. 75% received the vaccine “Sputnik V“, 25% received a placebo.
- Two doses were administered with a time interval of 21 days.
- 78 COVID infections have been observed, 16 among participants with the Sputnik V vaccine, 62 in the placebo group.
- Thus, the resulting efficacy is 92%.
- 11% of trial individuals were older than 60 years of age. The minimum age was 18.
- 20 cases of moderate and severe COVID appeared, all of those in the placebo group.
- The vaccination led to usual reactions as flu-like symptoms, local skin reaction, headache and fatigue.
Side effects were seldom and minor in both the vaccine (0,6%) and placebo group (0,6%). Deaths were also observed in similar numbers in both groups (<0,1%).
Conclusion
Results are very good: The efficacy is excellent, safety is provided.
Nevertheless, in a country like Russia it cannot be excluded that political influence has been exerted on scientists. All trial data were collected in only one city, Moscow. In comparison to that, data of the AstraZeneca trial were collected in numerous cities in the United Kingdom, South Africa and Brazil.
The assessment of the EMA (European Medical Agency) will be of utmost importance to me.
Judging by the trial design and working principle of the vaccine, the above mentioned results are realistic. Nevertheless, it would be helpful to gain data from countries with more reliable media. Once more, we witness how important it is to live in a free country based on the rule of law, also in non-political matters.

Saturday, January 30th 2021
Corona vaccine of Oxford/AstraZeneca: ChAdOx1 (AZD1222)
Corona vaccine of Oxford/AstraZeneca: ChAdOx1 (AZD1222)
an interim analysis of the above-mentioned study has been published on Jan 9th, 2021 (original publication). Since results are quite complex, I split up my article into a short version and a details part.
Short version
- Data of 11.636 individuals have been analyzed, one half received the COVID vaccine ChAdOx1, the other half not.
- 98 cases of COVID have been observed, 27 of those in the vaccine group, 71 in the other group.
- This results in a vaccine efficacy of 62%.
- 10 cases of COVID were hospitalised, two were severe, one patient deceased. All of those cases were in the group without the COVID vaccine ChAdOx1.
- Side effects were comparable to those of other common vaccines. One single case of an allergic reaction (anaphylaxis) has been observed.
Summary
The efficacy of the COVID vaccine ChAdOx1 seems to be lower than that of the mRNA vaccines of Biontech/Pfizer and Moderna, however with an efficacy of 62% WHO requirements of > 50% are met.
A vaccine with a moderate efficacy is better than no vaccine at all, especially if this vaccine is able to prevent hospitalisations and deaths. Thus, it serves its purpose.
Final results remain to be seen. Until then, this vaccine is an option for individuals with 18-55 years of age, but rather as a second choice in comparison to mRNA vaccines.
Time will show how frequently this vaccine will be administered. It will depend on what other vaccines will be available and what their efficacy will be.
Details for interested readers
- One half of individuals received the COVID vaccine ChAdOx1, the other half either a meningitis vaccine (serotypes ACWY) or a placebo.
- Unintentionally, a subgroup of inidividuals received only half the vaccine dosage with the first shot (“low dose group”, 2.741 indidivuals), while the majority received the full dosage (“standard dose group”).
- In the standard-dose group 98 cases of COVID were reported, thereof 27 in the ChAdOx1 vaccine group and 71 in the comparison group. Thus, the resulting efficacy of the vaccine is 62%.
- In the low-dose group 33 cases of COVID were reported, thereof 3 in the ChAdOx1 vaccine group and 30 in the comparison group. Thus, the resulting efficacy of the vaccine is 90%.
- In all 11.636 analyzed cases there were 10 cases of COVID that were hospitalised, two were severe, one deceased. All of those cases were in the comparison group without the COVID vaccine ChAdOx1.
Side effects
- Unfortunately, there is (still) no detailed data of typical vaccination effects like injection site pain, joint pain or fever.
- Side effects in the COVID-vaccine group were comparable to those in the other group. Sorted by organ systems, there were 84 compared to 91 cases. Sorted by side effects of special interest, there were 95 compared to 126 cases.
- In summary, the ChAdOx1 COVID vaccine group showed less side effects than the comparison group. A single allergic reaction (anaphylaxis) to the ChAdOx1 vaccine (among 12.021 vaccinated individuals) should be mentioned.
Commentary
- 88% of cases were 18-55 years of age. The publication clearly states that “… vaccine efficacy in older age groups could not be assessed but will be determined, if sufficient data are available…”. Therefore, in my opinion the German vaccination board’s (STIKO) recommendation not to approve the vaccine for older individuals is well-founded.
- In 2.741 individuals, the interval between the two doses was 69 days instead of 28 days as planned.
- It is not yet clear why the efficiency of the lower dosage seems to be better that the standard dosage. To understand that, it is necessary to know the working principle: The vaccine contains a chimpanzee common cold virus (ChAd1) that carries the genetic information of the SARS-CoV-2 spike protein. Normally, our immune system does not know this virus (ChAd1). As soon as it “sees” it for the first time with the first vaccination shot, antibodies against ChAd1 will be produced. One explanation of immunologists: The lower the vaccine dosage, the lower the level of antibodies. Thus, less vaccine of the second shot will be neutralized by antibodies, therefore the second vaccination will work better. For those reasons, AstraZeneca has started a cooperation with the Gamaleya institute in Russia. They use two different viruses to avoid this adversary immune effect (Ad26 for the first, Ad5 for the second shot).
- AstraZeneca has pooled data of individuals with the lower and with the standard dosage, and thereby calculates a resulting efficacy of 70%. But since individuals will either receive the low or the standard dosage and nothing in between, I personally doubt whether such a proceeding is permittable.

Wednesday, January 13th 2021
A Corona vaccination makes sense for everyone
A Corona vaccination makes sense for everyone
if we think about the question whether it makes sense to vaccinate against Corona or not, there are two points to think about:
- What will happen if I’m vaccinated? More precisely, what risks does the vaccination imply?
- What will happen if I’m not vaccinated? More precisely, what risks does the disease imply?
To answer these questions, we will look at data from two large clinical trials with 35.000 vaccinations: The Biontech/Pfizer vaccine trial and the Moderna vaccine trial.
No side effects worth mentioning in 35.000 vaccinations
One allergic reaction in 100.000 vaccinations after approval
After the approval of the first two vaccines, allergic reactions have happended in about 1 in 100.000 vaccinations (review article). Such an allergic reaction has to be taken seriously. Swift measures have to take place (injection of anti-allergic drugs). Nevertheless, an allergic reaction can be taken care of quickly and in most cases easily. No further consequences have to be expected.
Vaccine damage hypothetically in one of one million vaccinations
Anti-vaccinationists often stoke fears about vaccine damages. But those damages are much more seldom than they make them appear: In the years 2005-2009 about 40-50 million “non-private" patients have been vaccinated in Germany. The number of patients with a vaccine damage was around 40 per year (original data).
Thus, the number of vaccine damages is 1 in 1 million vaccinated individuals, based on vaccines of the past. In all current Corona vaccines, no vaccine damages whatsoever have been reported yet.
Mortality risk and health damage caused by COVID-19
Patients > 80 years of age have the highest risk: 1 of 7 infected with COVID decease. Patients 60-80 years of age decease in 1 of 28 cases, 35-59 years of age in 1 of 556 cases. Young individuals 18-34 years of age also have a relevant risk: 1 of 10.000 cases decease.
One important consequence of a COVID-19 infection is seldom mentioned: People who went through COVID partly need weeks, months or even years to recover. This “long COVID” or “chronic COVID syndrome” affects up to 10% of patients (review).
A permanent health damage is to be expected when pulmonal damage caused by COVID is so severe that invasive ventilation is needed. Quality of life and life expectancy will certainly be affected in these cases, but probably also in less severe cases. In the coming years, we will have more precise data, but we have to act now.
Among 42 COVID cases in my practice, 12% suffered from an asthma-like pulmonal reaction with the need for treatment (asthma spray). None of those patients had a history of a pulmonal disease. 5 patients were hospitalised, one patient deceased.
Direct comparison of risks
All numbers are preliminary and therefore approximations, definite numbers will probably need years.
Even if the worst possible vaccine is used (efficiency 50%), there will still be saved at least 9.000 lives. In comparison to that, we will probably have just one case of a vaccine damage among those 1.000.000 vaccinated individuals (no cases of vaccine damage reported yet).
Summary
Lethality in individuals aged 70+ is 1:28 up to 1:7, but even young individuals under 35 years of age still have a lethality that is ten times higher than the probability of the worst side effect of a vaccination: an allergic reaction.
Long-term health damage caused by COVID will keep us occupied for the coming months and years.
Thus, answering our question is very easy: Get vaccinated!

Sunday, January 3rd 2021
Publication of the Corona Vaccine Trial: Moderna „mRNA-1273“
the original data of the “second” Corona vaccine has now been published on December 30th 2020 (original publication).
Results:
- 30.420 individuals were included, one half (14.134) received the verum (true vaccine), the other half a placebo (14.073), a minority was excluded. The minimum age was 18 years.
- 185 cases of COVID-19 were observed in the placebo group and only 11 cases in the verum group, resulting in an excellent efficiency of 94% of the vaccine. Thus, efficacy is equivalent to the Biontech/Pfizer vaccine.
- 30 cases of severe COVID-19 were observed in the placebo group, one individual deceased, whereas in the verum group no cases of severe COVID-19 or deaths were reported.
Further details:
- 2.2 % of individuals had already suffered from COVID-19 before receiving the vaccine. No complications were observed in those cases.
- Panel A shows the frequency of local reactions like pain at injection site, redness and swelling.
- Panel B shows the frequency of other side effects: fever, headache, fatigue, muscle pain, joint pain, nausea and vomiting, chills.
- In summary, side effects were very similar to those observed in the Biontech/Pfizer vaccine and other common vaccines.
- No allergic reactions were observed since the number of test individuals was too small. A later analysis estimates the (preliminary) incidence of allergic reactions at 1:100.000 (original publication)
- The vaccine can be stored at 2-8°C (36-46°F).
- However, these data cannot show whether the vaccination will prevent an asymptomatic infection. Consequently, a vaccinated individual will be protected from a severe course of infection with a very high probability, but if an individual will contract the coronavirus, he might still be contagious to others despite the vaccination. Therefore, wearing a mask will still be necessary to protect those who can’t receive the vaccine, e.g. children, pregnant women and individuals with severe immune deficiency.
- Long-term side effects can’t be excluded; however, I personally agree with the assessment of vaccine specialists that the risk of a health damage from an infection with COVID-19 will outnumber any long-term side effects of the vaccine.
Conclusion:
The Moderna vaccine is highly efficient and safe. It protects from a severe COVID-19 infection much better than expected (94% achieved, 50% demanded by WHO). Side effects are in the usual range.
Results are excellent; thus, I have no objections to this vaccine.

Sunday, December 13th 2020
Publication of the Corona Vaccine Trial: Biontech/Pfizer „BNT162b2“
after the announcement of preliminary data about the above-mentioned vaccine in a press release on November 18th, the original data has now been published officially on December 10th (original publication).
This publication confirms the data from the press release:
- 43.661 individuals were included, one half received the verum (true vaccine), the other half a placebo.
- 162 cases of COVID-19 were observed in the placebo group and only 8 cases in the verum group, resulting in an excellent efficiency of 95% of the vaccine.
We have now more detailed data about side effects:
- 37,706 participants provided at least 2 months of safety data to assess side effects. 49% were female, 21% had a least one coexisting condition, 42% of participants were older than 55 years of age.
- Panel A shows the frequency and severity (in percent of participants) of pain at injection site, redness and swelling, respectively for individuals 16-55 years of age and above 55 years of age.
- Panel B shows the frequency and severity of other side effects: fever, fatigue, headache, chills, vomiting, diarrhoea, muscle pain, joint pain, respectively for individuals 16-55 years of age and above 55 years of age.
- In summary, side effects were very similar to those observed in other common vaccines.
- However, these data cannot show whether the vaccination prevents an asymptomatic infection. Consequently, a vaccinated individual will be protected from a severe course of infection with a very high probability, but if an individual will contract the coronavirus, he might still be contagious to others despite the vaccination.
- Long-term side effects can’t be excluded; however, I personally agree with the assessment of vaccine specialists that the risk of a health damage caused by an infection with COVID-19 will vastly outnumber any side effects whatsoever of the vaccine.
Conclusion:
The Biontech/Pfizer vaccine is highly efficient and safe. It protects from a severe COVID-19 infection much better than expected (95% achieved, 50% demanded). Side effects are in the usual range.
Further data will be collected. Until further notice, I have no objections to this vaccine and will report about my personal experience as soon as I will be vaccinated.
Estimations are that it will be protective 3 weeks after application. A vaccinated individual will probably be protected for at least several months, possibly up to 1.5 years.

Sunday, November 1st 2020Topic: SARS-CoV-2 Vaccines ("Corona Vaccine")
Currently 180 Corona virus vaccines are being developed world-wide. This text analyzes seven of these specifically addressing the following:
1. Whether it is technically possible to cut short the vaccine development by several years without a loss of safety of the vaccine?
2. Which vaccines are being developed now? What results are known about efficacy and safety?
1. Acceleration of the development of a Corona virus vaccine
a) Design and testing in animal models: This process usually takes years and could be almost entirely skipped since there is already sufficient data from the development of vaccines for coronaviruses in 2003 (SARS) and 2012 (MERS).
For economic reasons you often find delays: The studies have to be funded, and that takes time. Moreover, a pharmaceutical enterprise has to check time and again whether the investment into the next phase still makes sense, regarding the actual market conditions and a demand for the product.
Thus, phase I to III studies usually take 5-7 years.
Current proceedings are different:
- Huge fundings and investments from governments worldwide are available.
- The three phases are being planned in a cascade instead of one after the other. Normally, such a proceeding would pose an unacceptable economic risk: If a serious problem arose in an earlier phase so that the study had to be cancelled, all investments into later phases would be lost. Thanks to governmental funding, the cascade planning can take place without a loss in quality.
- Otherwise competing companies are now cooperating and sharing resources and tasks, thus saving time in development and testing.
Prof. Krammer stresses that an acceleration of the vaccine development process is mainly possible by taking high financial risks. Nevertheless, no intolerable compromises are being made about the safety of the vaccine.
If intolerable side effects were observed, it would mean the end of a vaccine. Therefore, pharmaceutical companies work with the utmost care not only because of ethical, but also because of simple economical reasons not to lose the market to rival companies.
2. Description of seven potential vaccines under development
Inactivated vaccine (inactivated SARS-CoV-2) with aluminium hydroxid as adjuvant. Two doses were administered with 2-4 weeks in between. The safety profile (absence of serious adverse effects) was described as excellent. 90% of test individuals showed antibodies against corona, thus proving a working vaccine within that time period. However, antibody levels were quite low, and younger individuals had higher levels than older ones. The phase III trial has started.
b) Company: Sinopharm.
Inactivated vaccine (inactivated SARS-CoV-2) with aluminium hydroxid as adjuvant. Two doses were administered with 2 or 3 weeks in between. Antibody levels were in the average range. Side effects were comparable to CoronaVac (see above). The phase III trial has started.
c) Company: CanSino
That vaccine contains an adenovirus (harmless common cold virus), that has been modified so it will not be able to propagate. I will carry the SARS-CoV-2 spike protein.
The spike protein is the structure on the coronavirus’s surface which enables it to enter a human cell. The immune system recognizes that spike protein and produces antibodies against it.
A single dose of this vaccine was administered. Antibody levels were low. The following side effects were observed: Fatigue and headache, in 50% of cases pain at the injection site, fever in 9% (high vaccine dose) respectively 1% (low vaccine dose). The phase III trial has started.
Explanation: Replication-incompetent adenoviruses will be recognized and destroyed by the immune system. This adenovirus has no means to defend itself let alone to harm the human body since the genes it needs to replicate have been removed.
Thus, the adenovirus is just a carrier that presents the spike protein to the immune system so it can produce antibodies.
This vaccine type has been successfully applied against Ebola in central Africa.
d) Company: AstraZeneca
Again, this vaccine uses an adenovirus, that is unable to propagate and will carry the SARS-CoV-2 spike protein.
The majority of individuals received a single-shot, the minority a second dose after 28 days. Antibody levels of the single-shot group were in the average range while they were higher in the group with a second dose.
Common side effects were fatigue (> 70% of individuals) and headache (> 60% of individuals). Fever or a feverish sensation have also been observed.
The phase III trial has started.
e) Company: Moderna
A new vaccine type has been implemented: Lipid nano particles (LNPs, “lipid bubbles”) contain an mRNA, that works as a “construction plan” for a protein, in this case the spike protein of SARS-CoV-2.
Background knowledge: In human cells, mRNA is a non-identical, but same-sense copy of a DNA sequence (genes in the cell's core). The mRNA is being transported to ribosomes outside the cell’s core but still within the cell. Those ribosomes produce the protein that is described in the mRNA’s “instructions”. After completing the protein, the mRNA will be dissolved. (see Fig. 3).
Operation principle of an mRNA vaccine: LNPs transport the mRNA into the body and the cell where the mRNA finds its way to the ribosomes. After ribosomes produce the spike protein, the mRNA is being dissolved. The spike protein is recognized by the immune system which will lead to the development of antibodies. After that, the spike protein will also be dissolved.
The spike protein cannot do any harm without the rest of the virus. This mRNA is just a construction plan and no virus. It doesn’t have any contact with the cell's DNA let alone influence on the DNA.
mRNA vaccines are also being developed for use against Zika virus and CMV virus.
About the Moderna vaccine: Two doses were administered with 4 weeks in between. Antibody levels were in an average to high level, comparable to levels found in individuals who recovered from COVID-19. In contrast to vaccines with inactivated virus, beyond the production of antibodies even a T-cell response could be proven. This is relevant since a broader and sustained immune response can be expected. Thus, an individual with such a vaccine will probably have a better and longer protection against SARS-CoV-2.
Regarding side effects, fever has been reported in 40% (after first shot) and 57% (after second shot).
The phase III trial has started.
f) Company: Pfizer / Biontech (Mainz)
Again, LNPs (Lipid nano particles) are being used to transport an mRNA into the body. In this case, the mRNA is a “construction plan” just for the receptor binding part of the spike protein (RBD, receptor binding domain) and not the whole spike protein.
Two doses have been administered with 3 weeks in between. Average to high antibody levels were observed which were comparable to patients who recovered from COVID-19. Fever has been reported as a side effect.
A second vaccine from Pfizer is in development, also using LNPs and mRNA, but the mRNA codes for a complete spike protein. In this case fewer side effects were observed.
The phase III trial has started.
g) Company: Novavax
A different vaccine type is in use here: A complete spike protein is produced in insect cells and afterwards inserted into a micelle (kind of globule) (original article). Parts of the protein will still be “visible” to the immune system on the micelle’s surface.
Two doses were administered with 3 weeks in between. Only after adding an adjuvant, relevant and very high antibody levels could be observed. In addition to that, a T cell response could be detected. As mentioned before, this might produce better and longer lasting protection against coronavirus.
Side effects included: fatigue, malaise, headache, pain at injection site, muscle ache, joint ache, nausea, vomiting, fever.
The phase III trial has started.
Concluding assessment: SARS-CoV-2 vaccines
All mentioned above vaccines trigger antibodies of a certain type called “IgG”. These antibodies are capable of protecting the lower airways (lungs). Unfortunately these vaccines don’t lead to a sufficient production of “IgA” antibodies that mainly protect the upper airways (throat, nose, sinuses).
Therefore, such vaccines will only protect from a severe infection of the lungs but not from an infection of the upper airways. Consequently, I expect that face covers will still be necessary even after receiving a vaccine.
The development of a live, attenuated vaccine that will be applied via the nose (exists for influenza) would have the advantage that it can trigger the production of either IgG and IgA. Unfortunately those vaccines are in a very early stage of research and have not yet made it to clinical trials.
At the moment it is still unknown how well older individuals will response to a vaccine. Furthermore, older patients need higher antibody levels than younger ones for a sufficient protection (experience from influenza research).
Prof. Krammer concludes that the vaccines developed by AstraZeneca, Moderna and Pfizer will have a sufficient efficacy with a high probability, thus an approvement of those vaccines is to be expected provided that side effects remain minor.
I would like to add that serious complications of vaccines are generally overestimated and have to be clearly distinguished from common side effects like headache, fatigue, joint pain and fever. In the last 9.5 years we have administered around 12,000 vaccination without a single complication and just the usual side effects. We forget too often that millions of lives are being saved and harms to our health avoided by vaccinations: The measles vaccine has saved 17 million lives in the years 2000-2015 (WHO about measles). And only a vaccine could eradicate smallpox that hat cost 300 million lives in the 20. century (WHO about smallpox).
Finally, I personally believe that the development of a corona vaccine will be remembered as a unique and unprecedented international cooperation and achievement. We will probably owe participating scientists and other persons involved our respect and gratitude.

Sunday, July 26th 2020
Dear patients,
at the moment we can enjoy a quite calm time in Germany regarding the Corona pandemic. This gives us the opportunity to recover from the stress, enjoy the nice weather and finally meet again with friends – of course in compliance with safety rules (find more on CDC.gov).
In case there will be an increase of cases this autumn, our motivation to isolate ourselves again will certainly be better if we will have used this summer as well as possible. For psychological reasons I therefore regard it as very important to allow oneself this little break from the pandemic.
Today I would like to address these topics:
- Corona warning app (in Germany)
- Face masks
There is still no ground-breaking news about vaccines, antiviral drugs, antibody tests and antigen tests. Results simply need time. Also there is currently no evidence of a crucial influence of virus mutations (see original article) and the blood group of patients (see original article).
Corona-Warn-App
For more information about the source code of this app and data protection issues please look in heise.de (in German).
Face masks
Mainz University and others have shown how infections have been avoided by wearing masks in the German town of Jena (see diagram), this analysis is easy to understand (look here).
There are multiple other trials with the same findings, e.g. examining the situation in Italy (see second diagram).
A quite graphic example has been reported about in the Washington Post and also in Der Spiegel: Two hairdressers had continued to work in their salons, unknowing about their Corona infection. Obviously thanks to wearing masks, not a single infection has been observed in 140 of their clients.
Interested readers can find a more complex but very detailed analysis in The Lancet.
3 million saved lives in Europe

Sunday, June 21st 2020
"Corona-warn-app"
Dear patients,
since Tuesday the „Corona-warning-app“ („Corona-warn-app“) is ready for download. This application for smartphones is meant to warn the user after close contact with people within the previous two weeks who have registered themselves as Corona positive. In such a case the user can put himself into a voluntary quarantine.
The application is sufficiently safe regarding data protection (see article at heise.de). The source code (the „programming“) is open source so every expert can look at details and functions. Please find instructions of use at heise.de.
This app gives the population the chance to get local outbreaks quickly under control without the need for extensive restrictions and lockdowns.
The recent outbreak in a meat processing factory near Gütersloh could probably have been stopped much earlier with such an app and might have avoided infecting over 1000 people.
Thus, I recommend the use of this app and of course installed it on my personal smartphone. There is good scientific evidence for the advantages of such an app (see original article).
You can download the app here.

Monday, June 1st 2020
Dear patients,
today I would like to address these topics:
- Current situation of the corona pandemic in Germany
- Presentation of information by the media
- „R value“ and comparing corona virus with influenza
- The COVID-19 infection – what do we now yet?
Current situation of the corona pandemic in Germany
This is a very positive result, thanks to the majority of sensible citizens. They succeeded in neither overestimating nor underestimating the threat posed by the corona virus. Contrary to that, the behaviour of a minority on demonstrations is dangerous: The spreading of false information leads to wrong behaviour and thereby endangers the lives of others.
There has been an outbreak of corona virus infections in a retirement home in Wiesbaden. This shows that it isn’t over yet.
Presentation of information by the media
Science is a slow process that needs time and patience. But the media needs fast information and sensation, otherwise they might be ignored. This contrast leads to the misunderstanding that there are conflicting recommendations following each other in a short time.
Therefore, I recommend not to take every little news as confirmed knowledge but to wait until facts from trials confirm or contradict that information.
Nevertheless I would like to stress that it makes sense to get your information from trustworthy main stream media and newspapers. Please do not turn away from that media and get your information from self-pointed “gurus” on YouTube or rumours on Facebook, WhatsApp, Twitter etc. This will only lead you astray. You might also ask someone on the street how to treat corona virus.
Please find reliable information here:
https://www.coronavirus.gov
https://www.cdc.gov/coronavirus/2019-nCoV/index.html
„R value“ and comparing corona virus with influenza
The left axis shows the lethality. As shown on the diagram, Ebola is more dangerous than SARS-CoV-2.
The diagram also shows that it doesn’t make any sense to say “corona is (just) as dangerous as a flu”.
Influenza can have a lethality that differs very much from one season to the other. The “bird flu” is extremely dangerous and comparable to Ebola while the 2009 flu was as harmless as a common cold.
SARS-CoV-2 is shown as a read area instead of a dot because we still lack precise data.
Nevertheless, the lethality of corona virus is high enough to make health care collapse especially if the threat isn’t taken seriously.
The COVID-19 infection – what do we now yet?
In the second stage the virus reaches the lungs. A throat swab will often turn out negative for SARS-CoV-2 now although the patient has the virus. Shortness of breath can appear. The patient has the typical signs of a pneumonia.
The third stage is characterized by an over-reaction of the immune system. As a consequence, the body is harmed by itself. Therefore, there are ongoing trials to find a way to slow down the immune system in that phase of the infection.
Moreover, in stage III inflammations of the myocardium are found, possibly leading to cardiac failure. Also thrombosis and pulmonal embolisms appear, caused by a hypercoagulation.
This is what is known yet about COVID-19. Other observations are only preliminary. The media tend to present such observations as confirmed facts, but this is not the case. For example, there had been reports about certain syndromes in children. But it is much too early to assess that situation. Two observations might appear at the same time without a causal link, and quite often in science there will be following data that will contradict the first data.
Therefore, I recommend to wait patiently and let science do its job. (Find diagram paper here)

Sunday, May 15th 2020
Dear patient,
I would like to talk about several topics today:
- Staying smart in the pandemic
- How to know where the virus comes from?
- Is Corona really that “bad”?
- Why is mortality so different in various countries?
- Why does it take so long with developing a vaccine?
- Anything new about the antibody test?
- Is there a medication for Corona yet?
- Live your lives! – with social distancing, masks and hygiene
Staying smart in the pandemic
In an exceptional situation as this Corona pandemic it is of utmost importance to keep a cool head, use our brain and get our information from safe sources: from experts.
This is the best way to manage our lives in such times since it will last on until a vaccine will be available. Neither panic nor negation are helpful.
How to know where the virus comes from?
Every virus has its genetic fingerprint. If the fingerprints of the Corona virus in different countries worldwide is being compared with the ones found in different animals, the source can be identified.
Such fingerprints are shown on the diagram. The green line shows a virus endemic in bats, the red line shows the worldwide pandemic virus in humans. As you can see, the fingerprints of those two viruses are mostly identical. Little differences are normal since viruses change in their cycle of propagation.
Thus the origin of the virus can be regarded as identified.
Is Corona really that “bad”?
Numbers are abstract, it’s difficult to have an image in your mind. Therefore, let me give you a report from real life: I receive reports from befriended physicians in the Rhein-Main area, Germany and the whole of Europe. They are on the “front line”, working in hospitals, partly on intensive care. I studied with many of them and know their competence and reliability.
All of them tell me that the current situation is much different from what we know of past years and decades of influenza and other epidemics. Depending on the region they live in, there is a much higher flow-in of patients. Capacities are partly under stress (Germany), but partly medical care is not possible anymore for all incoming patients (London). A makeshift hospital with 4000 beds had to be built. (Article on Business-Insider).
COVID-19 patients are much longer on intensive care and ventilated longer that those with an influenza or common pneumonia, thus capacities of beds and respirators are blocked longer than usually.
There are antibiotics to treat bacterial pneumonias and Oseltamivir to treat influenza, but with SARS-CoV-2 (“Corona”) there is no comparably efficient drug. Doctors can only wait and hope.
A befriended married couple of doctors in London sends me dramatic reports. The inflow of patients is extremely high, a normal medical care is not possible anymore. There aren’t enough beds, respirators, medical staff.
When she (internist) tells me that three nurses known to her in the age of mid-fifties died in her hospital and another six nurses and physicians survived on intensive care, there is no doubt to me that the situation is very serious and we don’t ask too much from people to simply wear masks.
It would prove fatal to believe that this couldn’t happen to Germany. We can be thankful that doctors and politicians acted much earlier than in the United Kingdom or the US where the existence of a threat had been doubted in highest positions in politics even when the pandemic was already fully running. Consequences are obvious: Those two countries have witnessed a sheer explosion of infections while in Germany there are “only” minor outbreaks.
The whole world is lucky that Corona is “only” as bad as a “severe influenza without a vaccine”. If we had to deal with an Ebola pandemic, our masks would prove more or less useless and mortality could be around >68% (see last Ebola outbreak).
Why is mortality so different in various countries?
There are several reasons for that:
- Number of tests: In Germany, mildly sick patients have been tested while in Italy only moderate to severely sick patients received a test. The more mildly sick patients you have, the lower the mortality rate.
- Access to a test: In the US, for numerous week you had to pay for a test while in Germany it has been and is for free.
- Way of life: In Italy, many people live in larger families. Thus, the elderly is being infected by younger generations. In Germany we rather find small families so the elderly is “isolated” from the others.
- Hospital capacities: The number of beds, respirators and medical staff has been sharply reduced in the last couple of years in the United Kingdom and Italy. This has been done to safe expenses. Germany has also reduced capacities, but not to that extent as in above mentioned countries.
Last not least many patients are afraid to enter a hospital although it might be necessary. And inside hospitals the focus is shifted on Corona so there is a risk that other diseases get out of sight.
Why does it take so long with developing a vaccine?
There are no Corona vaccines from past experiences so there is no basis to build on. This is a crucial difference from the situation with influenza vaccines: They can be designed and produced within months.
Nevertheless, parts of other vaccines can be used as a basic element and modified to work as a Corona vaccine. Thus, the development of a Corona vaccine will (hopefully) not take 5-10 years as this is the usual average time for a vaccine development.
Testing a vaccine for efficiency and tolerability takes most of the time. A vaccine that is being tested in Mainz will be used in healthy volunteers for several months. After that, it will be tested in older volunteers with pre-existing conditions.
Some people erroneously believe that one day they will receive an untested vaccine. Nothing could be more wrong: E.g. in Mainz a first vaccine is already there but due to the need of thoroughness and safety, such a vaccine will be tested for a long time. In other words: Only after making sure that a vaccine has been tested thoroughly enough for efficiency and safety, it will be offered to the public.
As soon as such a vaccine will be available, I expect it to be distributed to risk groups first, just as in the swine flu pandemic in 2009.
Anything new about the antibody test?
At the moment, I do not recommend to have an antibody test for Corona. The information about the reliability of that tests stems from the company that produces it. The data base for reliability calculations has been rather small (69 Corona patients, see diagram).
Moreover, antibodies do not necessarily have to be protective antibodies: Patients P1 to P10 did have antibodies, but those antibodies couldn’t neutralize the virus (no visible columns on the diagram). The rest of patients (P11 to P175) showed different levels of neutralizing potency from way below 500 up to 20.000.
In certain situations, antibodies can prove harmful: After having gone through Dengue fever, you might develop antibodies that will make a later, second infection with Dengue virus even much worse. Thus, antibodies are not always the same.
Lastly, an immunity against a disease is not only caused by antibodies but many others parts of the immune system including “trained” T-type lymphocytes. And that makes the situation more complicated.
Long story short:
- If a patient has a positive antibody test, he doesn’t know if he will be immune to Corona virus, thus he has to continue to protect himself by wearing a mask etc. since he can still be infected.
- If a test turns out negative, the patient has to protect himself by wearing a mask etc.
Since the result is the same, I do not recommend doing a test now. And remember that you would have to repeat such a test every week. My recommendation will certainly change in the future, but not today.
If someone still wants to do such a test, I expect a test centre to be opened at the end of May, but costs have to be paid by the patient himself.
I strongly advise against thinking that a patient will be immune because of a positive antibody test. If someone visited his parents or grandparents without the necessary protection, this could have fatal results for them.
Is there a medication for Corona yet?
Remdesivir has the best data. In a US study (see diagram) it showed a certain efficacy that lead to its approval in the US a week ago while this step still being evaluated in Europe.
Remdesivir alone is not efficient enough. More substances are being tested.
Live your lives! – with social distancing, masks and hygiene
Since the Corona pandemic will last on for months, presumably another year, we can’t remain in a state of emergency because of psychological reasons. We need a certain amount of normality, otherwise the stress will be too much for us.
On the one hand, we need to follow protective measures without even thinking about them because they have become daily routine: distancing, masks, washing hands. On the other hand, we need to get back to our daily routine and way of life. Thus, we can feel that life goes on.
Research is working hard to find solutions. This might me invisible for non-physicians. New substances are being developed, trials performed to find out more about how the virus spreads so we will have more precise information how to protect us and at the same time have more freedom. And vaccine trials are on the way.
Nevertheless this “bad dream” will only be over when we receive a vaccine. I will keep a close eye on those developments. Initially I spent 10 hours a week with online presentations and conferences on Corona virus, at the moment it is only a couple of hours per week. This way I can continue to seek out the most important information of this complex topic, summarize it and put it in a shape that can be easily understood.